Nickel titanium alloy, also known as nitinol, is used in a wide range of coronary and vascular stents that help treat coronary artery disease, peripheral artery disease and other conditions. In this Q&A, MTS Senior Applications Engineer, Scott Anderson discusses the research he and his colleagues are pursuing to deepen the industry’s understanding of this material’s fatigue life.
Q: What is the focus of your research and why is it important?
Anderson: The paper my colleagues and I are developing focuses on the effects of mean strain on the fatigue life of nickel titanium alloys (NiTi). Many implantable medical devices such as endovascular stents (stents), stent-grafts and heart valve structures are made from nitinol. Each of these products experiences a range of mean strains as they are shaped and heat-treated during manufacture, compressed for deployment and implanted in the patient. Their location in the body affects the mean and alternating strains they experience during their functional life. To optimize the design of new devices, manufacturers need a better understanding of how these strains affect the fatigue life of the alloy.
Q: How is fatigue life testing traditionally done in these applications?
Anderson: Prior to nitinol, the primary material had been 316L stainless steel. Nitinol has only been used to produce self-expanding stents since the mid ‘90s. Regulatory agencies such as the FDA have not accepted traditional fatigue life models for nitinol devices and have required each new design to be validated by an envelope of 10-year-equivalent life tests (400,000,000 cycles per test) encompassing all the possible loading conditions including a range of mean strains each with a range of alternating strains. The result is hundreds and possibly thousands of 400,000,000 cycle tests to prove the device will not fail for the intended use.
Q. What unique challenges do nitinol device manufacturers encounter?
Anderson: Because these structures and their applications are relatively new, there is little historical data available than for other materials, such as stainless steel. Since the material behaves so differently than traditional steels, traditional fatigue life modeling has not proved predictive for failure. This lack of available predictive modeling means that medical device manufacturers have to provide more data, which affects their costs and time to market. The Shape Memory and Superelastic Technologies (SMST, a special interest group of the ASM) was formed in the late ‘90s to address the concerns of nitinol device manufacturers.
Q: What effect does mean strain have on device design?
Anderson: The combination of increasing mean strains with larger alternating strains can directly lead to fracture. Fractured endovascular stent structures might penetrate arterial or even coronary walls creating undesirable conditions (internal bleeding) for a patient. To illustrate this challenge, consider an endovascular stent that is implanted in an artery above the knee. It’s going to be compressed and twisted hundreds of times a day as a result of rising, walking, squatting, stair climbing and simple pulsatile distension. Therefore, these stents need to be extremely durable.
Q. What are some of the greatest measurement challenges?
Anderson: The diversity of devices creates challenges in material characterization. The medical device industry is finding more and more uses for nitinol as new devices are invented. In addition, there are several ways to manufacture these devices. Some stents are laser cut, others are woven. Some are bare metal, others are covered in fabric. Pre-straining can be part of the process for shape memory devices, but this may increase or decrease fatigue life depending on the particular stent architecture. What engineers are trying to determine is whether the material can be used to create the design they have in mind, and what the tradeoffs will be in terms of uniformity and flexibility. All of this affects design time and therefore cost.
Q: What do you hope to achieve with this research?
Anderson: Ultimately we want to help the medical device industry create better, more durable devices that improve patients’ quality of life for longer periods at a lower total cost. Right now, these devices are subject to intense scrutiny during the FDA approval process. The FDA focuses on all the potential modes of failure, structural failure being a key one. To gain approval, manufacturers have to simulate all of the mechanical conditions the device is subject to and often they need to produce mean strain data. Typically, they are required to provide 10 years of fatigue life data for each mechanical condition to achieve a 90% confidence ratio; and just one of these tests requires 400 million cycles.
Q: How do these requirements impact time and cost?
Anderson: The tests are very expensive. Manufacturers have to run hundreds of these tests for a single device. Running at 60 Hz, it takes about 80 days to complete 400 million cycles if the test system is operating 24/7. Ideally, manufacturers want to find a way to minimize the total test time. That is the intent of our research. Together with SMST and ASTM, we have a technology committee that includes materials manufacturers, device manufacturers, clinical specialists and application engineers. We are working to develop fatigue-to-fracture standards for nitinol that will allow manufacturers to get more and better information about fatigue life from less testing.
Q: Do the current requirements have other implications for test labs?
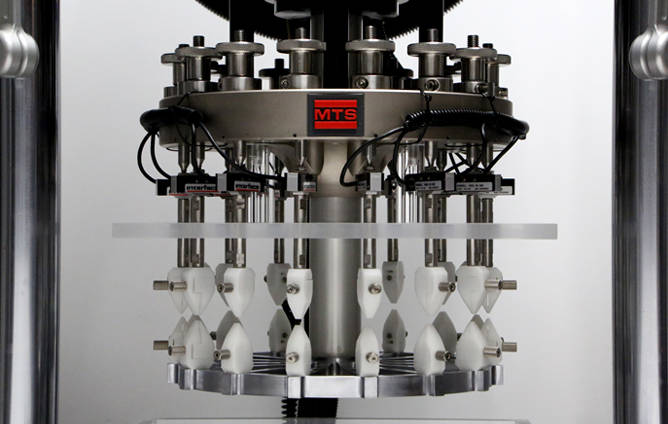
Anderson: Due to the total number of cycles, high frequencies and displacements of about 400 microns, conventional servohydraulic mechanical test systems are not an ideal choice for this application. At MTS, for example, we recommend using the MTS Acumen™ Electrodynamic Test System. Its design is well-suited to these kinds of tests because it can perform high-frequency testing with relatively little energy output and very little wear on the machine.
Q: Are you using the MTS Acumen system for your current research?
Anderson: Yes, we are using the MTS Acumen system to generate our data. Specifically, we are taking straight sections of wire that have been pre-strained, clamping them in place and then applying an alternate strain. The MTS Acumen system is very good at this kind of test. Also, we are using a bath to keep the specimen below 37°C, because as strain is applied and heat accumulates we do not want to trigger the grain structure shift that makes shape memory possible in the first place.